IRIS CRO is a full-service Clinical Research Organization established by experienced clinical research professionals. We deliver high-quality, client-focused operational support across Latvia, Georgia, Poland, Lithuania, and Ukraine, ensuring speed, compliance, and predictable results for global sponsors. Our multi-country footprint allows us to support studies with regional expertise, strong site networks, and fast regulatory timelines.
We consistently meet deadlines, regulatory requirements, and enrollment milestones across all therapeutic areas and study phases.
Our leadership team unites experts with extensive industry backgrounds, committed to driving operational excellence and delivering successful clinical trials.
What we stand for:
Full-service clinical operations across 5 countries
ICH-GCP compliant processes and strong quality system
Experienced CRAs, SSU & Regulatory specialists, and oversight monitors
Fast start-up timelines in Eastern Europe
Transparent communication and proactive issue resolution
Flexible, sponsor-focused, solution-driven approach IRIS CRO is dedicated to delivering reliable, agile, and high-quality clinical research — supporting sponsors from biotech, pharma, and medical device industries worldwide.
IRIS CRO is expert in Pharmaceutical, Biotechnology, Medical Device and Cosmetics industries, offers high quality research service.
Our customers Manager - Rita Iesalniece, IRIS CRO Director, co-founder.
▶ 20 + year experience in clinical research area.
▶ 1 year experience in Syneos Health Georgia as Assoc. Director, Clinical Operation Manager, responsible for Georgia, Armenia, Lebanon, Turkey.
▶ 9 year experience as AmberCRO foreign branches Director, responsible for Georgia and Armenia
▶ 9 year Clinical Monitoring experience.
▶ More than 20 year as Internal Diseases Specialist experience in Latvia.
▶ 14 year participation experience in clinical trial in Latvia mostly as Principal Investigator.
▶ Education: Riga Medical Academy, Latvia, MD, RISEBA University, Latvia, MBA.

Our services
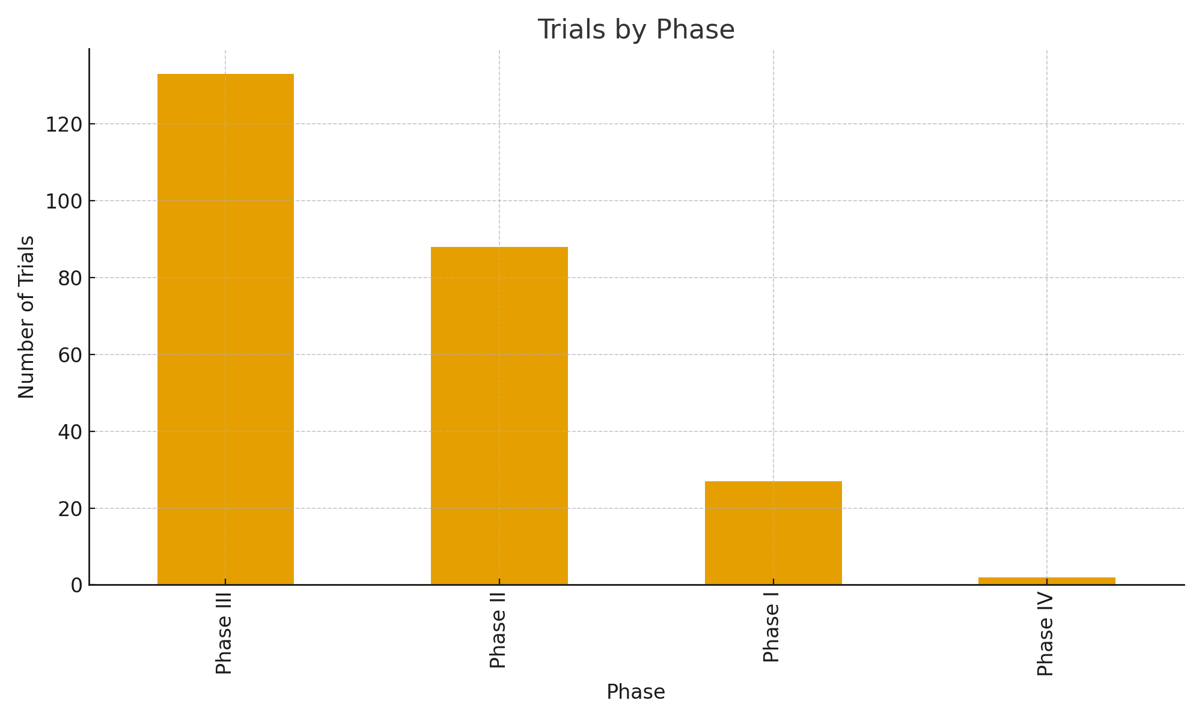
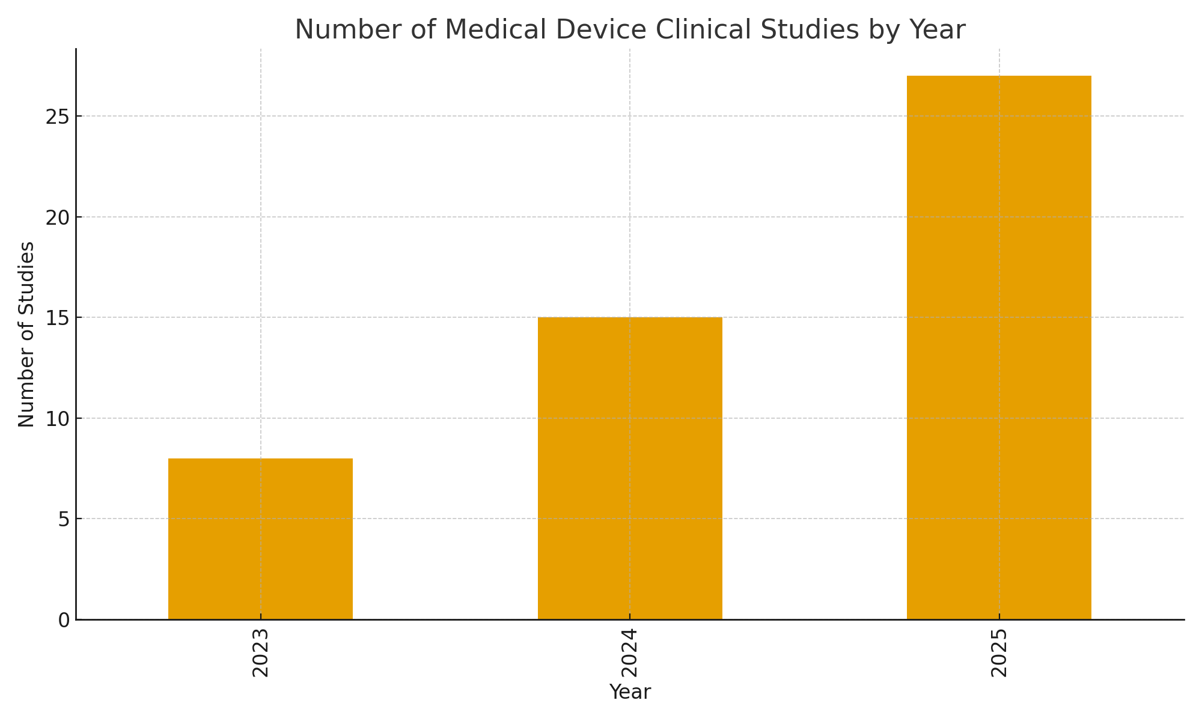
Our service for clinical trials phase I-IV in in Pharma, Biotech, Medical Device and Cosmetics industries
Feasibility
Accurate, rapid, professional country/site-level feasibility assessment is essential for successful clinical trial progress, completion.
IRIS CRO has huge country level databases with experienced,
motivated, high recruitment potential sites. Databases updates on daily basis. Only sites with experienced staff, acceptable enrolment, high patients' retention and data quality can be considered as suitable.
Site Selection
Choosing the right site is critical to a successful clinical trial. Sites must have an accurate history of accurately patient enrolment in the required quantity, site staff and procedures must be professional to ensure proper patient retention, sites must be able to provide quality data.
Site Start up
IRIS CRO highly professional Start-up &Regulatory Specialists with more than 5 years of experience are well educated in country regulatory requirements, meet deadlines, helping the sponsor work with Regulatory Authorities and Ethics Committees, coordinating translations and negotiate contracts with sites by following timelines.
Clinical Monitoring
IRIS CRO Clinical Research Associates are highly professional, with more than 3-4 year experience in clinical trials, are experienced in Cardiology, Pulmonology, Oncology, Surgery, Rheumatology, Dermatology, Neurology, Infectious Diseases, Hematology, Endocrinology, Medical Devices, Cosmetics, are assigned for projects by their experience, demonstrate high levels of site management skills. Company’s management follow CRAs training development process to be sure to have the high-level service.
IRIS CRO ‘has dedicated procedures and an experienced team for unblinded monitoring, including managers who manage the parts of trials related to unblinded drug management, and CRAs with vast experience in both onsite and remote unblinded monitoring.
Project management
IRIS CRO provides project management service, has experienced Project managers who directly works with clients, lead clinical studies, support the planning, set up and coordinate processes of clinical trials, develop and manage budgets, meet deadline and quality standards.
Medical Writing for medicines and medical devices
We can provide Study design and study outline development, development of Clinical Investigator Plan (CIP) for medical devices: pilot, pivotal, and postmarketing studie, Phase I-IV Clinical Study Protocol development and amendments to them, Informed Consent Form (ICF) development, Investigator's brochure development, Case Report Form (CRF) creation.
Data management services
Data management planning (Data Management Plan, Data Validation Plan), EDC system management (OnlineCRF or other EDC’s): configuration, validation, , edit-check programming, account management, etc., performing User Acceptance Test, Data review and data cleaning (medical monitoring, identification of the data discrepancies and outliers, cross-checks, logic checks, query generation/resolution), performing risk-based monitoring, pre-DB closure activities, soft lock, hard lock, Datasets formation and assembly, Blinded Review Meeting preparation and conduct.
Vendor Management
IRIS CRO is experienced with the third party Vendors selection such as customs clearance service, depot service, translation of medical text any complexity, transport patients.
Regulatory Service
IRIS CRO regulatory specialists are high-level professionals available to provide exceptional services, are with excellent knowledge of regulatory documents preparation/submission processes, with successful outcomes.
Flexible Resourcing services
IRIS CRO provides experienced and professional staff for all company’s services.
Quality
For IRIS CRO every client is unique. Quality management is one of the main aspects of the work of IRIS CRO. All company’s staff receives trainings in regular basis on high priority. Our clients can be sure of the high quality of provided service. Company is working by following applicable international regulatory requirements and guidelines (i.e. ICH-GCP, and/ or EU-Directives).
We are responsible for
▶ Organization of clinical trials I- IV in Pharma, Biotech, Medical Device and Cosmetics industries
▶ Purchase, storage and distribution of pharmaceuticals
▶ Customs clearance service
▶ Translation of medical texts of any complexity
▶ Transport of patients.
We offer portfolio of services which can be tailored to meet the individual needs of each client.
Timelines for Initial submission in IRIS CRO countries:
| Pharmacology | Medical devices | Comments2 | |
|---|---|---|---|
| Georgia LECs | 3-4 weeks | 3-4 weeks | |
| Georgia RA | 21 working day for review+15 working days for queries answering | 21 working day for review+15 working days for queries answering | LEC approval document is part of RA initial submisison package |
| Latvia EC | EUCTR submission via CTIS validation 10 days + 15 days for query resolution, 45 assesment + 31 days for query resolution. Max 106 days | 1 month for review, in case of additional questions time is stopped untill answers are provided to EC | submission of documents takes place in parallel to EC/RA. Project can be started when both approvals are available |
| Latvia RA | EUCTR submission via CTIS validation 10 days + 15 days for query resolution, 45 assesment + 31 days for query resolution. Max 106 days. | 1 month for review, in case of additional questions time is stopped untill answers are provided to RA | submission of documents takes place in parallel to EC/RA. Project can be started when both approvals are available |
| Ukraine EC | 4 weeks | 4 weeks | submission of documents takes place in parallel to EC/RA. Project can be started when both approvals are available. |
| Ukraine RA | 1 month + additional time for queries resolution | 2 weeks + additional time for queries resolution | submission of documents takes place in parallel to EC/RA. Project can be started when both approvals are available. |
| Poland EC | EUCTR submisison via CTIS , max 106 days including queries resolution | 1 month + additional time for queries resolution | submission of documents takes place in parallel to EC/RA |
| Poland RA | EUCTR submisison via CTIS , max 106 days including queries resolution | 1 month + additional time for queries resolution | submission of documents takes place in parallel to EC/RA |
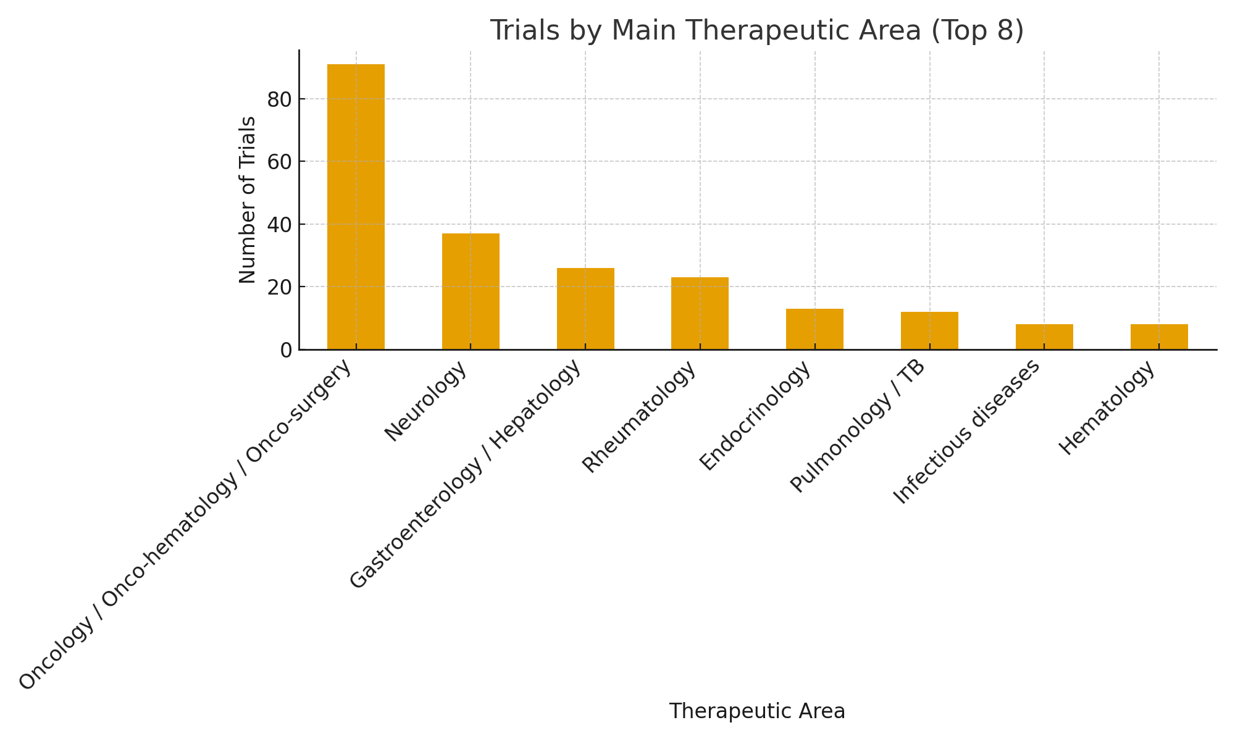
Therapeutic Areas
Cardiology
Pulmonology
Oncology
Rheumatology
Dermatology
Neurology
Infectious Diseases
Hematology
Endocrinology
Surgery
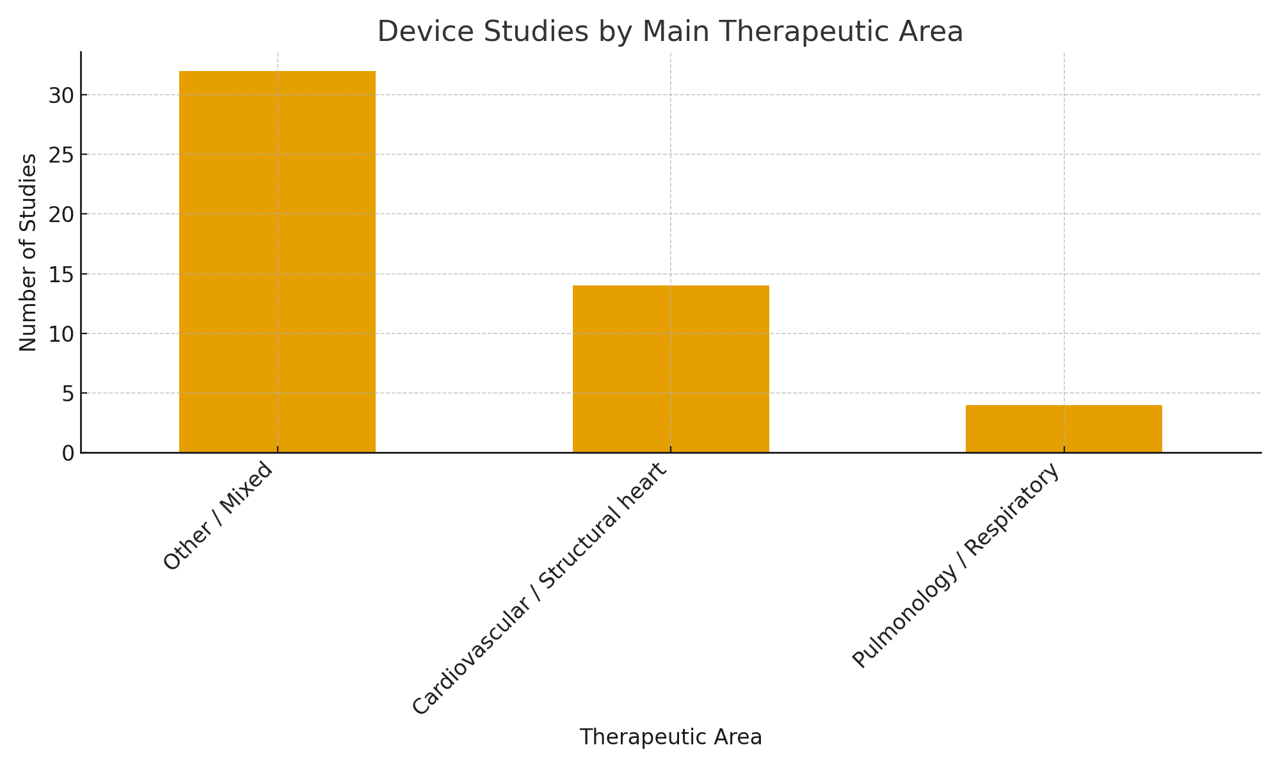
Medical Devices